Edentulous jaw is a condition where either the upper (maxilla) or the lower (mandible) jaw is missing all teeth. In medical practice, it could be treated by placement of a complete denture.
Previous research has already pointed that the application of a border molding procedure (or functional shaping) results in significantly fewer cases of pressure ulcers (decubitus) and soft tissues deformations, hence increased retention and stability of the prosthesis, both at rest and in function. Since there are many factors that affect the optimal treatment, such as anatomical structures (i.e. muscles, muscular and soft-tissue gripping) and the asymmetry between the left and right halves of upper and lower jaws, it is important that special care is taken to determine the depth, as well as the width of the tissue where the teeth would normally be nested (gingivobuccal sulcus). With border molding, it is possible to determine those, however, the accuracy of the impression would still largely depend on the materials used in the procedure.
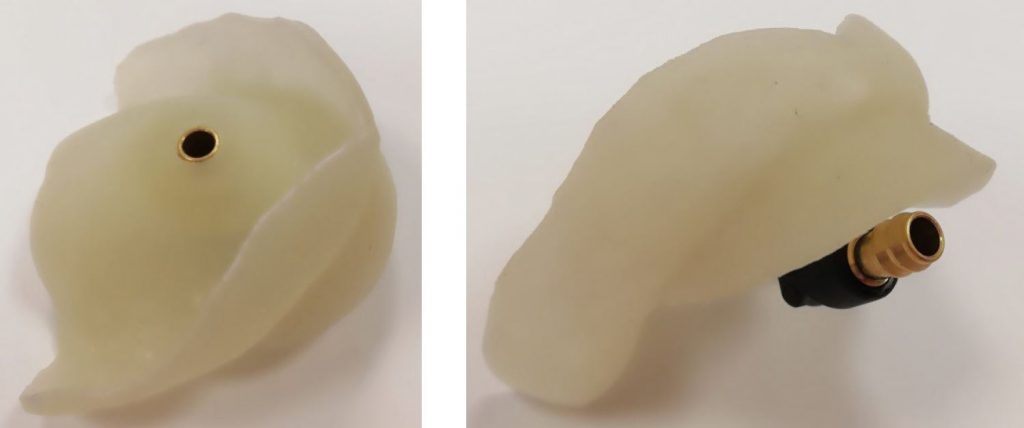
Image: Dobromira Shopova, PhD
In their study, published in the open-access, peer-reviewed scholarly journal Folia Medica, Dr Dobromira Shopova and Prof. Diyan Slavchev at the Plovdiv Medical University (Bulgaria) sought to evaluate and determine the accuracy of two different groups of impression materials for border molding: thermoplastic and elastomers. They examined four different brands: Detaseal function (additive silicone for border molding), Sta-seal F (condensation silicone for border molding), GC Iso functional sticks (synthetic resin for border molding), Kerr Impression compound green sticks for border molding.
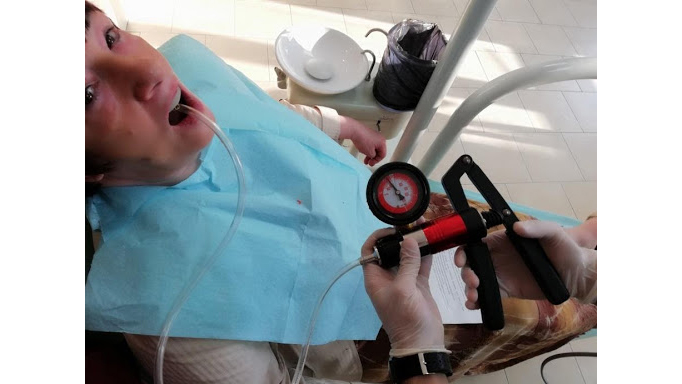
To perform their research, the team applied Dr Dobromira Shopova’s clinical method to measure negative pressure after border molding procedure, referred to as the vacuum measurement technique on edentulous upper jaw. They also assembled a special custom tray from a light-curing base plate with a palatal adapter. This was a 900, 7-millimetre metal adapter, which was fixed to the midline on the palatal slope. To create and measure the negative pressure, they used a combined pressure pump. The maximum value was 3 bars for positive pressure and -1 bar for negative pressure.
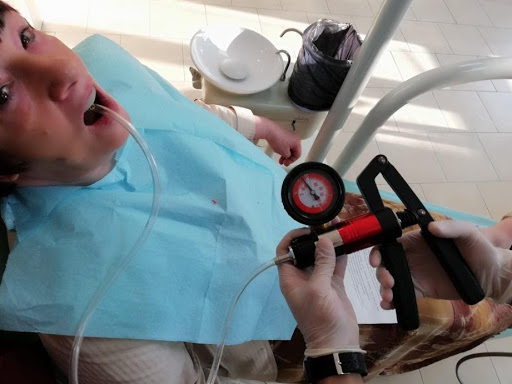
Image: Dobromira Shopova, PhD
Working protocol followed for all materials:
1. Apply the impression material along the edge of the individual tray;
2. Insert, position and perform Herbst functional tests;
3. Wait for the elasticity or hardening of the material;
4. Assemble the clinical unit for negative pressure measurement;
5. Measure the negative pressure that has been created between the custom tray and the prosthetic field, then record the result;
6. Release the individual impression tray from the patient’s mouth.
A statistically significant difference was observed between the two thermoplastic materials: the GC Iso functional sticks and the Impression compound green sticks. No statistically significant difference was observed between the other groups of materials.
The measured mean negative pressure values created between the prosthetic field and the custom tray showed close values for each patient – with a difference of -0,05 to -0,1 bar. This showed that the anatomical features of the prosthetic field were of great importance.
In conclusion, quantitative measurement of negative pressure is entirely possible under clinical conditions. Thermoplastic materials for border molding are retained and formed only along the edge of the custom tray. However, silicone impression materials do not spread only on the edge of the custom tray, but also on the alveolar ridge, demonstrating their superior manipulative qualities and accuracy for the purposes of border molding.
Original source:
Shopova DA, Slavchev D (2020) Clinical Negative Pressure Measurement after Border Molding Procedure. Folia Medica 62(3): 578-584. https://doi.org/10.3897/folmed.62.e48464